The Novavax COVID-19 vaccine trialled in Northern Ireland has been approved for use
Nuvaxovid, the COVID-19 vaccine developed by Novavax, has been given regulatory approval by the Medicines and Healthcare products Regulatory Agency (MHRA).
It becomes the fifth COVID-19 vaccine authorised by the UK’s independent medicines regulator. In reaching its decision, the MHRA considered the results of 2 large clinical trials involving nearly 50,000 participants.
In Northern Ireland almost 500 participants from across the region volunteered to take part in the clinical trial which was led by researchers Professor Danny McAuley, Professor Judy Bradley and Dr Johnny Stewart from Queen’s University Belfast and the Belfast Health and Social Care Trust.
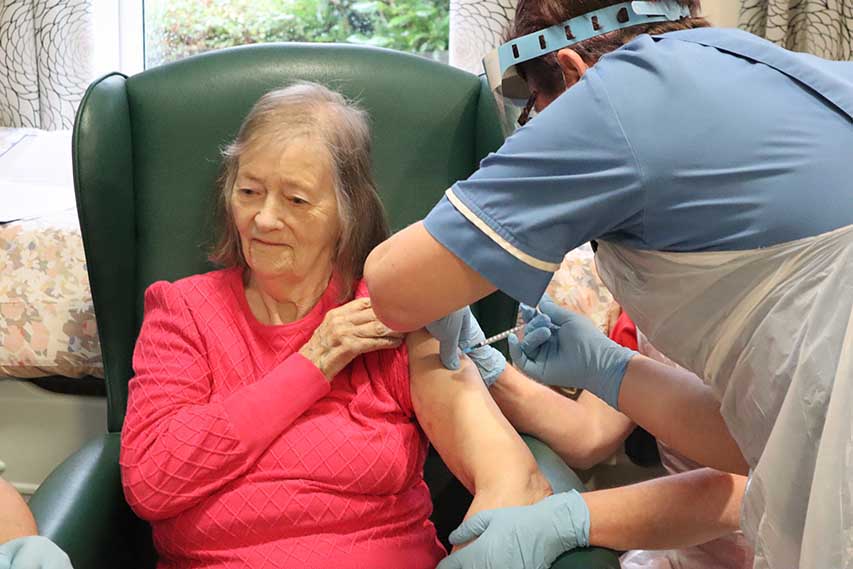
A new Covid-19 vaccine has been trialled in Northern Ireland and given approval.
A number of Health and Social Care and academic organisations and individuals supported the trial, including the Public Health Agency R&D Division, the Belfast Health and Social Care Trust, NI Clinical Research Facility, the NI Clinical Research Network, Queen’s University Belfast and Ulster University, involving medical nursing and administrative staff from across Northern Ireland as well as medical, nursing and physiotherapy students, community GPs and Belfast COVID Centre staff.
Welcoming the approval of the Novavax vaccine, Dr Janice Bailie, Assistant Director of HSC R&D Division Public Health Agency (PHA), said: “This is another positive step forward in the battle against COVID-19.
“Belfast Trust was one of a group of hospitals across the UK to deliver the Novavax trial, in which almost 500 participants from Northern Ireland volunteered to take part.
“As funder of the Northern Ireland clinical R&D infrastructure, we are extremely grateful to each and every one of the participants, and also to the research team who worked so hard to deliver the study.
“There is no doubt that research has made a huge contribution to the development of tests, treatments and vaccines to protect the health of the population during this most difficult of times. Looking ahead, it is important that investment in research is maintained for the future so that evidence will be generated to guide the post-pandemic recovery process.”
This was the first COVID-19 vaccine trial to take place in Northern Ireland and the number recruited to take part in trial surpassed the original target of 350.
John Conlon volunteered to take part in the Novavax trial. He said: “It’s easy to see how the world changed for most of us in the UK after March 2020. We all wanted things to get back to ‘normal’, and when the adverts for the trial came up, I thought it was something I could do to help out.
“The staff were excellent in the NI Clinical Trial Unit, helpful at explaining the trial and what was involved. It’s great to see the vaccine has been approved for use and hopefully we are seeing some light at the end of the tunnel that has been the past two years”.
The Novavax study was the largest double blind, placebo-controlled COVID-19 vaccine trial to be undertaken in the UK so far. The Belfast site was one of a number across the UK to host the trial, which investigated the safety, efficacy and immunogenicity of NVX-CoV2373 – a stable, prefusion protein antigen derived from the genetic sequence of the SARS-CoV-2 coronavirus spike (S) protein and adjuvanted with Novavax’s proprietary Matrix‑M™.
Gordon Kennedy, Novovax trial participant said: “Having been involved for many years assessing research grant applications as a lay member of the Alzheimer’s Society research network, I decided to volunteer as a participant in the Northern Ireland cohort for the Novavax trial.
“As a first time trialist, I am delighted to learn that the Novavax vaccine has now been fully approved for use in the UK. Hopefully, this will enable many more vaccinations to take place on a worldwide basis.
“I would like to thank the team at QUB Clinical Trial Centre for the way they looked after us, both during the injection processes and in their thorough follow up procedures, ensuring that any potential side effects were examined and recorded. Happily, I had none. I would simply add my congratulations to the whole research community for their amazing work in providing vaccines for us all”.
More information about taking part in research and other opportunities to take part in COVID-19 research can be found at: